- ALL COMPUTER, ELECTRONICS AND MECHANICAL COURSES AVAILABLE…. PROJECT GUIDANCE SINCE 2004. FOR FURTHER DETAILS CALL 9443117328


Projects > ELECTRONICS > 2020 > IEEE > DIGITAL IMAGE PROCESSING
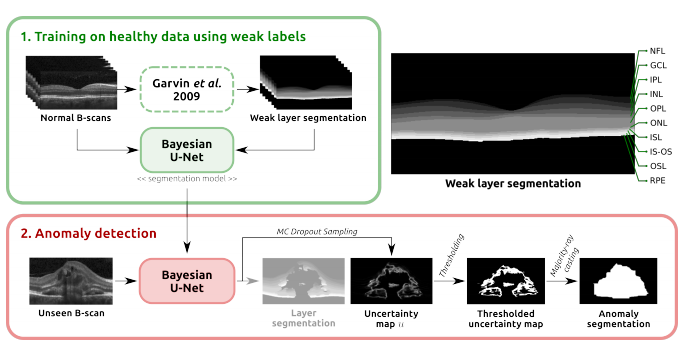
Diagnosis and treatment guidance are aided by detecting relevant biomarkers in medical images. Although supervised deep learning can perform accurate segmentation of pathological areas, it is limited by requiring a-priori definitions of these regions, large-scale annotations, and a representative patient cohort in the training set. In contrast, anomaly detection is not limited to specific definitions of pathologies and allows for training on healthy samples without annotation. Anomalous regions can then serve as candidates for biomarker discovery. Knowledge about normal anatomical structure brings implicit information for detecting anomalies. We propose to take advantage of this property using bayesian deep learning, based on the assumption that epistemic uncertainties will correlate with anatomical deviations from a normal training set. A Bayesian UNet is trained on a well-defined healthy environment using weak labels of healthy anatomy produced by existing methods. At test time, we capture epistemic uncertainty estimates of our model using Monte Carlo dropout. A novel post-processing technique is then applied to exploit these estimates and transfer their layered appearance to smooth blob-shaped segmentations of the anomalies. We experimentally validated this approach in retinal optical coherence tomography (OCT) images, using weak labels of retinal layers. Our method achieved a Dice index of 0.789 in an independent anomaly test set of age-related macular degeneration (AMD) cases. The resulting segmentations allowed very high accuracy for separating healthy and diseased cases with late wet AMD, dry geographic atrophy (GA), diabetic macular edema (DME) and retinal vein occlusion (RVO). Finally, we qualitatively observed that our approach can also detect other deviations in normal scans such as cut edge artifacts.
Gaussian Mixture Model (GMM)
First, we train a Bayesian U-Net model on normal cases to segment retinal layers, using weak labels automatically generated with a graph-based segmentation approach. Secondly, this model is applied together with Monte Carlo dropout to retrieve pixel-level epistemic uncertainty estimates. Finally, we introduce a simple post-processing step, majority-ray-casting, to transform the uncertainty maps into compact segmentations of anomalies. This technique closes the gap between the shape of layers and anomalies based on the assumption that anomalies in OCT are compact and not layered. Therefore, using retinal layer information is an appropriate way of incorporating anatomical knowledge into the model. At the same time, no labels of the target class (i.e. anomalies) are needed for training.
BLOCK DIAGRAM